The group of the month I would like to introduce to you today is a group I have a close personal connection with. The group goes back a long way with me. I will present you the annelid family Dinophilidae.
After my Diploma thesis (today Master thesis) on proteins of a virus related to HIV at the Max Planck Institute of Molecular Physiology in Dortmund I wanted to do a different topic for my PhD. I never really connected with the topic of my Diploma thesis and that made me think about my passion in biology. I recognized that my reason why wanted to study biology was about evolution and zoological systematic. This really got my fired in on all my intellectual pistons. Hence, I reached out to Prof. Westheide, head of the Zoological Systematic group at the University of Osnabrueck, and he suggested different possible PhD topics including one studying meiofauna on the Galapagos islands. However, the one subject, which really triggered me, was about progenetic evolution of Dinophilidae and similar taxa.
What are Dinophilidae?
Dinophilidae are so-called interstitial species. This means they are so small that they can move in the space between the sand grains without moving them. Their body length ranges from 1 to 3 mm, but in Dimorphilus species the males are dwarf males and are as small as 50 µm. Hence, these Dimorphilus dwarf males are the tiniest animals on Earth. They are essentially only a sac, which is full of sperm, with very few nerve cells (about 50) and a copulatory organ. With the organ, they inject the sperm into the body of the female, which is often their own sister within the shared egg cocoon. Hence, their only purpose in life is to provide sperm and to die afterwards.
The body organization of dinophilids, which are not dwarf males, is relatively similar and they always have a head followed by a segment with two rings around the mouth, then six trunk segments and the final body unit with the anus called pygidium in annelids. The pharynx of the mouth is a massive muscular bulb, which has a unique organization of their muscle cells. Usually in animals, the fibrils in the muscle cells are responsible for the movement of the muscle cell and are orientated only in one direction. The fibrils can actively only be shortened, but for extending them another component (e.g., another muscle cell) needs to stretch the muscle cell and with this the fibrils in it. In this pharyngeal muscle bulb are the fibrils orientated in two directions perpendicular to each other. The effect is that these muscle cells cannot be shortened by the action of the fibrils as the two sets of fibrils work against each other. This sounds like a disadvantage, but actually causes the pharyngeal muscle bulb to become a very stiff organ allowing to scrap organic material from surfaces.
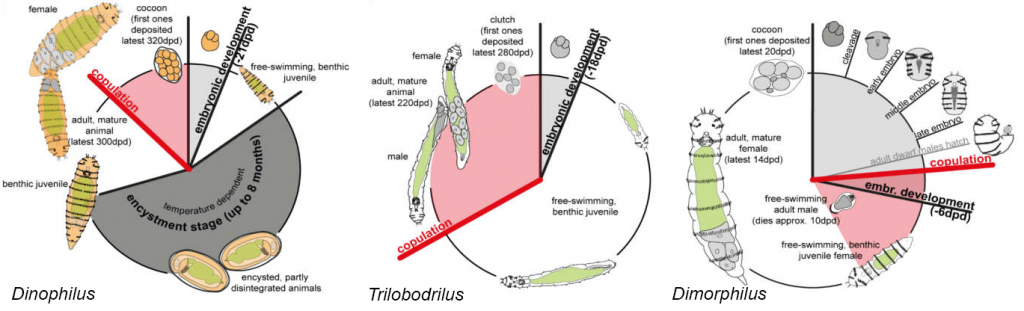
The family comprises three genera: Dinophilus with five species, Dimorphilus with two species and Trilobodrilus with eight species. The life cycle of Dinophilus species takes about a year, which includes three to four weeks of embryonic development and obligate stage of encystment of up to eight months. Similarly, the life cycle of Trilobodrilus species is also approximately a year including the embryonic development of two to four weeks. However, a resting stage like in Dinophilus is lacking. In contrast, the life cycle of Dimorphilus is much faster with about three weeks for the females including 6 days of embryonic development. The life cycle of the dwarf males is even faster with about one week.
What is progenetic evolution?
Gould introduced progenetic evolution (or progenesis) as one of the evolutionary processes occurring by changes of the relative speed of development of the non-reproductive versus the reproductive body parts (or more scientifically the somatic versus the reproductive development). Progenesis is one of the two processes leading to paedomorphosis, which means that the body of the descendant looks more like the body of an ancestral juvenile or larval stage than the adult ancestral one. In progenesis, this is accomplished by the acceleration of the development of the reproductive organs, while the speed of development of the non-reproductive organs remains unaltered. Hence, such species become sexually mature in the juvenile or larval stage and stop their further development. They look like juvenile or larval stages and also have a similar body size as these stages. In contrast, the other process resulting in paedomorphosis is neoteny. In neotony, the development of the non-reproductive body parts (or parts of them) is decelerated, while the reproductive development occurs at normal speed. Such species also look like ancestral juvenile stages or traits, but have the body size of the adult stage. Humans have supposedly such neotenic traits. An example is the human trait to play even as adults.

How come progenetic evolution and dinophilids together?
Given their simple body organization, dinophilids were first thought to be flatworms, but then they were recognized as annelids. However, again given the simple organization they were considered as showing the ancestral condition of annelids reflecting the evolutionary trajectory from simple to complex as so often assumed in animal evolution. And as so often, this assumption was wrong. Detailed morphological studies could show that they are actually secondarily simplified organisms, probably having evolved by progenesis to allow a faster adaptation to the interstitial realm.
Given their similarity to the larval stages of the order Eunicida, which includes the presence of two rings around the mouth, it was suggested that they belong to this order, specifically the family Dorvilleidae. This is very interesting as this order also comprises some of the largest annelid species on Earth such as the sand striker worm (watch a video of them here), which can be up to 6 m long. However, the exact placement of Dinophilidae within the family Dorvilleidae was uncertain.
Myself, dinophilids and progenetic evolution – a long story
Here is now where my story begins. The idea of my PhD thesis was to use molecular data to show where they are placed within Dorvilleidae and based on this placement investigate the process of their progenetic evolution in more detail. However, as so often in life, things do not turn out as planned. During my studies, it became more and more obvious that Dinophilidae are probably not part of Eunicida at all. Hence, I needed to start to look at the whole annelid tree of life and not only part of it. At the end of my PhD, I could only show that they are not eunicidans, but I could not place them more accurately in the annelid tree of life with confidence. In my thesis, I mentioned with respect to the morphological similarities that they also could be found in another annelid family Orbiniidae.
Given my extension into the whole annelid tree of life during my PhD, I continued to work on this the next years after my PhD as well as the placement of Annelida in the animal tree of life. For this, I used more and more data and sophisticated method eventually employing phylogenomic methods. This means I gathered genome-scale data to answer my questions. At that time, this meant not the entire genome, but the transcriptome. As this was still quite challenging for such small creatures I had to develop new methods to do so for other microscopic animal groups. After I could successfully apply these methods for such small organisms I went back to address the topic of my PhD thesis. And we were successful; we could final place Dinophilidae within the annelid tree of life. Interestingly, it appeared that they evolved by progenetic evolution from an orbiniid-like ancestor. Hence, my journey with dinophilids kind of came full circle since I finished my PhD more than a decade earlier. In the study, we could also show that a few other interstitial annelid groups also evolved by progenesis, while others evolved by step-wise miniaturization. Hence, two evolutionary processes drove the adaptation to the interstitial realm.
There I thought the question I set out to answer in my PhD was finally settled after more than a decade and then along came Martin-Duran and his co-authors in 2020. By publishing the first genome of a dinophilid, they also reanalyzed the placement of Dinophilidae and the other interstitial groups. While they confirmed our previous position for most of the interstitial groups, Dinophilidae were placed differently. Together with another interstitial family, Lobatocerebridae, Dinophilidae were placed as the sister to the majority of the annelid biodiversity, a group called Pleistoannelida and which also comprises both Orbiniidae and Eunicida. Hence, they most likely still evolved by progenesis in this scenario, but given this position we cannot pinpoint to the morphological appearance of this ancestor as we can with the other two placements; morphologically to Eunicida before and in our analyses to Orbiniidae. Even 25 years after the start of my PhD as of this year, the question still remains where to specifically place Dinophilidae in the annelid tree of life.
Accordingly, the other question is: Will I retire over this question, which started my academic career in earnest?
Three fun facts to remember:
- Dinophilid dwarf males are the tiniest animals on Earth and just live to have sex.
- Dinophilids are essentially larval stages of an annelid ancestor that became sexual mature. The worst case of a premature teenager one could think of.
- Dinophilids have fibrils in perpendicular orientation to each other within the same muscle cell of the pharyngeal bulb. This makes the bulb a very effective scraping organ.
Background reading on dinophilids:
- Martín-Durán, J.M., B.C. Vellutini, F. Marlétaz, V. Cetrangolo, N. Cvetesic, D. Thiel, S. Henriet, X. Grau-Bové, A.M. Carrillo-Baltodano, W. Gu, A. Kerbl, Y. Marquez, N. Bekkouche, D. Chourrout, J.L. Gómez-Skarmeta, M. Irimia, B. Lenhard, K. Worsaae, and A. Hejnol, Conservative route to genome compaction in a miniature annelid. Nature Ecology & Evolution, 2020. https://doi.org/10.1038/s41559-020-01327-6
- Purschke, G., Anatomy and ultrastructure of ventral pharyngeal organs and their phylogenetic importance in Polychaeta (Annelida) – I. The pharynx of the Dinophilidae. Zoomorphology, 1985. 105: p. 223-239. https://doi.org/10.1007/BF00311966
- Struck, T.H., A. Golombek, A. Weigert, F. A. Franke, W. Westheide, G. Purschke, C. Bleidorn, and K.M. Halanych, The evolution of annelids reveals two adaptive routes to the interstitial realm. Current Biology, 2015. 25(15): p. 1993-1999. http://dx.doi.org/10.1016/j.cub.2015.06.007
- Struck, T.H., K.M. Halanych, and G. Purschke, Dinophilidae (Annelida) is most likely not a progenetic Eunicida: Evidence from 18S and 28S rDNA. Molecular Phylogenetics and Evolution, 2005. 37: p. 619–623. https://doi.org/10.1016/j.ympev.2005.07.010
- Worsaae, K., A. Kerbl, M.D. Domenico, B.C. Gonzalez, N. Bekkouche, and A. Martínez, Interstitial Annelida. Diversity, 2021. 13(2): p. 77. https://www.mdpi.com/1424-2818/13/2/77
- Westheide, W., Progenesis as a principle in meiofauna evolution. Journal of Natural History, 1987. 21: p. 843-854. https://dx.doi.org/10.1080/00222938700770501
![]()
