Cover picture by Sue Flood
Last time, I wrote about how glacial-isolation caused the divergence of a small population of ringed seals in the Ilulissat Icefjord, West Greenland. The text ended with a final consideration on the importance of developing research plan to retrieve molecular data for Arctic species.
Human caused climate change has severely affected high-latitudes regions, leading to permanent alteration of environmental dynamics. Polar marine mammals are known to exhibit a broad ecological plasticity, meaning they are able to adapt to a vast range of environmental conditions. However, how these animals will react to the unidirectional and unprecedented changes brought by the ongoing climate crisis is still unclear. Assessments development is further hindered by such intraspecific diversity we still know very little of, with populations coming from different geographical areas that might show various responses to shifting habitat conditions.
The uniqueness of Kangia ringed seals derive from the fact that they evolved through time within a restricted habitat. As a species, ringed seals have however a much wider distribution ranging through the Arctic (Rosing-Asvid et al. 2023). Developing research programs in Polar regions based on direct observations is however a challenging task. Not only hostile conditions can be found, but animals usually have low population densities over vast areas. Molecular analyses become so irreplaceable tools to get valuable information on the natural history of these marine mammals that would otherwise be difficult to obtain.
To better understand this concept, lets travel from the Ilulissat Icefjord in Greenland to the opposite part of the world. We are now in Antarctica, and seals are still around. However, they are not so closely related to the ringed seals we met before. They belong to a different subfamily, Monachinae, which is mainly distributed in the Southern Hemisphere. If you have ever watched a documentary about penguins, there is a high chance you also saw a leopard seal (Hydrurga leptonyx Blainville, 1820). The species is recognizable, along with other key traits, by its elongated body covered by dark spots (Fig.1). Females are usually larger than males, reaching a maximum length of 3,8 m and a weight of 500 kg. Lastly, they are mainly found in the Southern Ocean, with sightings recorded from northern locations. However, if there is something that made leopard seals famous to the general public it`s their diet, which also includes other pinnipeds and seabirds (Rogers 2017). And a lot of Antarctic krill (Hocking et al. 2013), but maybe I will talk about it in the future

Fig.1: Leopard seal morphology (Figure by Pieter Folkens)
While being charismatic, leopard seals are still one of the least studied pinnipeds. As such, they were often excluded from Antarctic ecosystem modeling despite their relevant role as high trophic level predators. Getting more data on leopard seals would mean obtaining a better comprehension of Antarctic dynamics.
In a study published in 2023, the team of Arona N. Bender and colleagues employed molecular analyses focusing on the D-loop region of mitochondrial DNA extracted from 90 different leopard seals (see Bender et al. 2023). The obtained results highlighted moderate levels for both nucleotide and haplotype diversity, similarly to what has been observed for Weddell seals (Leptonychotes weddelli Lesson, 1826), which are the closest living relatives of leopard seals. Furthermore, the current female effective population size, limited so only to the individual successfully reproducing and contributing to the genetic diversity of next generations, was estimated at ~12.188 individuals. With an assumed 1:1 sex ratio, that would suggest a total effective population size of ~24.376 leopard seals.
However, genetic data retrieved from modern specimens are not only important to assess the current diversity, but also represent a valuable window on the species` past. It was so possible to infer past demographic trends for the species, with positive trends started during the Last Glacial Maximum between 26 and 19 thousand years ago. Similar high levels were maintained up to ~6000 years ago, after which leopard seals` populations started to decline in a trend still continuing in the modern era (Fig.2). What`s surprising are the high effective population size estimates retrieved for such a high trophic level predator with a long generation time. This could be explained by the ecological and behavioral plasticity for which leopard seals are known, enabling them to exploit a broad range of habitats and feeding resources. Despite this, the estimated demographic trend highlights the importance of sea ice for the species, with the timing of population expansions and declines mirroring changes in habitat availability.
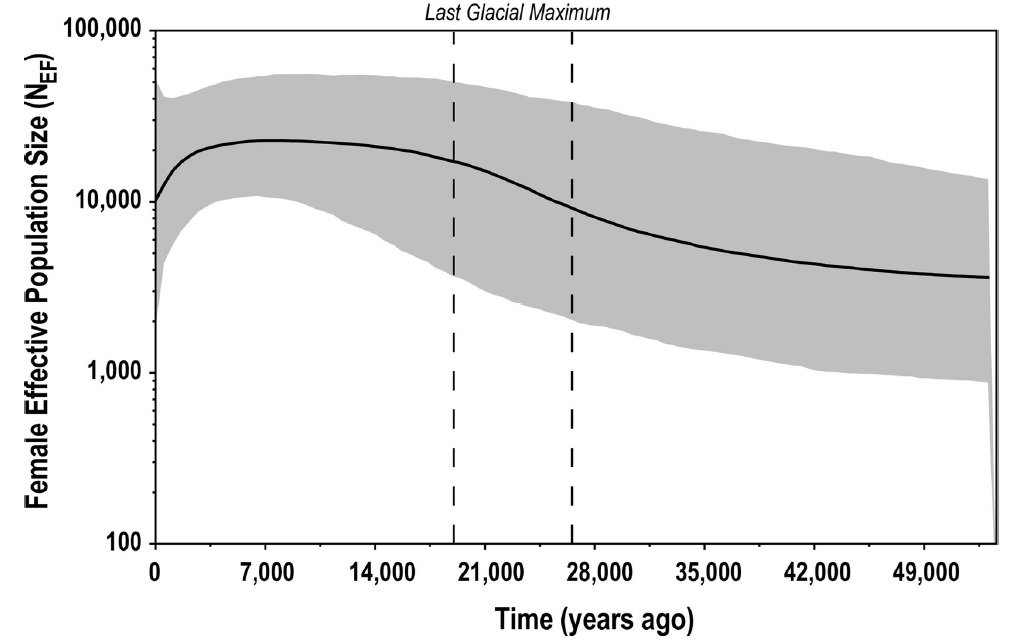
Fig.2: Bayesian Skyline Plot based on mtDNA sequences from 90 leopard seals. The timing of demographic expansion for the female effective population coincide with the Last Glacial Maximum (Picture from Bender et al. 2023)
As in a very strange Christmas carol, both past and present proved to be important to guess what the future might hold for leopard seals. As the annual sea ice cycle continue to deteriorate thanks to human activities, the species might lose a critical habitat for their life history. However, both sightings and reproduction evidence suggest the presence of resident leopard seals` populations along the coasts of New Zealand, which unlike Antarctica are pack-ice free (Hupman et al. 2020). Only new data will help to shed light on the ecology of this enigmatic predator.
References
Bender, A. N., Krause, D. J., Goebel, M. E., Hoffman, J. I., Lewallen, E. A., & Bonin, C. A. (2023). Genetic diversity and demographic history of the leopard seal: A Southern Ocean top predator. PLOS ONE, 18(8), e0284640. https://doi.org/10.1371/journal.pone.0284640
Hocking, D. P., Evans, A. R., & Fitzgerald, E. M. G. (2013). Leopard seals (Hydrurga leptonyx) use suction and filter feeding when hunting small prey underwater. Polar Biology, 36(2), 211–222. https://doi.org/10.1007/s00300-012-1253-9
Hupman, K., Visser, I. N., Fyfe, J., Cawthorn, M., Forbes, G., Grabham, A. A., Bout, R., Mathias, B., Benninghaus, E., Matucci, K., Cooper, T., Fletcher, L., & Godoy, D. (2020). From Vagrant to Resident: Occurrence, residency and births of leopard seals (Hydrurga leptonyx) in New Zealand waters. New Zealand Journal of Marine and Freshwater Research, 54(1), 1–23. https://doi.org/10.1080/00288330.2019.1619598
Rogers, T. (2017). Leopard Seal (Hydrurga leptonyx). In Würsig, B., Thewissen, J. G. M., & Kovacs, K. M., Encyclopedia of Marine Mammals, Third Edition (pp. 550 – 552). Academic Press;
Rosing‐Asvid, A., Löytynoja, A., Momigliano, P., Hansen, R. G., Scharff‐Olsen, C. H., Valtonen, M., Kammonen, J., Dietz, R., Rigét, F. F., Ferguson, S. H., Lydersen, C., Kovacs, K. M., Holland, D. M., Jernvall, J., Auvinen, P., & Tange Olsen, M. (2023). An evolutionarily distinct ringed seal in the Ilulissat Icefjord. Molecular Ecology, 32(22), 5932–5943. https://doi.org/10.1111/mec.17163
![]()
